In this step,we will find out the valence electrons of carbon.We have to know that the electrons of valence shell are called valence electrons. C(6)=1s² 2s²2p² From the above electron configuration of carbon,we see that carbon has 4 valence electrons in its valence shell. We know that the atomic number of calcium is 20.So calcium has 20 protons and 20 electrons as the charge of electrons and protons are equal but opposite in nature.The charge of proton is +1 and the charge of electron is -1. Step-3: Now write the electron configuration of calcium. Ca (20)=1s²2s²2p⁶3s²3p⁶4s².
Element Calcium - Ca
Comprehensive data on the chemical element Calcium is provided on this page; including scores of properties, element names in many languages, most known nuclides of Calcium. Common chemical compounds are also provided for many elements. In addition technical terms are linked to their definitions and the menu contains links to related articles that are a great aid in one's studies.
Calcium Menu
- Calcium Page One
- Calcium Page Two
- Calcium Page Three
Overview of Calcium
- Atomic Number: 20
- Group: 2
- Period: 4
- Series: Alkali Earth Metals
Calcium's Name in Other Languages
- Latin: Calcium
- Czech: Vápník
- Croatian: Kalcij
- French: Calcium
- German: Kalzium - r
- Italian: Calcio
- Norwegian: Kalsium
- Portuguese: Cálcio
- Russian: Кальций
- Spanish: Calcio
- Swedish: Kalcium
Atomic Structure of Calcium
- Atomic Radius: 2.23Å
- Atomic Volume: 29.9cm3/mol
- Covalent Radius: 1.74Å
- Cross Section (Thermal Neutron Capture)σa/barns: 0.43
- Crystal Structure: Cubic face centered
- Electron Configuration:
- 1s2 2s2p6 3s2p6 4s2
- Electrons per Energy Level: 2,8,8,2
- Shell Model
- Shell Model
- Ionic Radius: 0.99Å
- Filling Orbital: 4s2
- Number of Electrons (with no charge): 20
- Number of Neutrons (most common/stable nuclide): 20
- Number of Protons: 20
- Oxidation States: 2
- Valence Electrons: 4s2
- Electron Dot Model
- Electron Dot Model
Chemical Properties of Calcium
- Electrochemical Equivalent: 0.7477g/amp-hr
- Electron Work Function: 2.87eV
- Electronegativity: 1 (Pauling); 1.04 (Allrod Rochow)
- Heat of Fusion: 8.54kJ/mol
- Incompatibilities:
- water, oxidizers, acids, air, chlorine, chlorine tri-fluoride, fluorine, oxygen, silicon, sulfur
- Ionization Potential
- First: 6.113
- Second: 11.871
- Third: 50.908
- Valence Electron Potential (-eV): 29
Physical Properties of Calcium
- Atomic Mass Average: 40.078
- Boiling Point: 1757K 1484°C 2703°F
- Coefficient of lineal thermal expansion/K-1: 22E-6
- Conductivity
- Electrical: 0.298 106/cm Ω
Thermal: 2.01 W/cmK
- Electrical: 0.298 106/cm Ω
- Density: 1.55g/cc @ 300K
- Description:
- silvery, soft metal, tarnishes to grayish white after exposure to air.
- Elastic Modulus:
- Bulk: 17/GPa
- Rigidity: 7.4/GPa
- Youngs: 20/GPa
- Enthalpy of Atomization: 184 kJ/mole @ 25°C
- Enthalpy of Fusion: 8.54 kJ/mole
- Enthalpy of Vaporization: 150 kJ/mole
- Flammablity Class: Flammable solid
- Freezing Point:see melting point
- Hardness Scale
- Brinell: 167 MN m-2
- Mohs: 1.75
- Heat of Vaporization: 153.6kJ/mol
- Melting Point: 1112K 839°C 1542°F
- Molar Volume: 26.02 cm3/mole
- Physical State (at 20°C & 1atm): Solid
- Specific Heat: 0.632J/gK
- Vapor Pressure = 254Pa@839°C
Regulatory / Health
- CAS Number
- 7440-70-2
- RTECS: EV8040000
- NFPA 704
- Health: 1
- Fire: 1
- Reactivity: 2
- Special Hazard:
- OSHAPermissible Exposure Limit (PEL)
- No limits set by OSHA
- OSHA PEL Vacated 1989
- No limits set by OSHA
- NIOSHRecommended Exposure Limit (REL)
- No limits set by NIOSH
- Levels In Humans:
Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances.- Blood/mg dm-3: 60.5
- Bone/p.p.m: 170000
- Liver/p.p.m: 100-360
- Muscle/p.p.m: 140-700
- Daily Dietary Intake: 600-1400 mg
- Total Mass In Avg. 70kg human: 1 kg
Who / Where / When / How
- Discoverer: Sir Humphrey Davy
- Discovery Location: London England
- Discovery Year: 1808
- Name Origin:
- Latin: calx, calcis (lime).
- Abundance of Calcium:
- Earth's Crust/p.p.m.: 41000
- Seawater/p.p.m.: 390
- Atmosphere/p.p.m.: N/A
- Sun (Relative to H=1E12): 2240000
- Sources of Calcium:
- Obtained from minerals like chalk, limestone & marble. Very abundant. Makes up 3.5% of crust. Occurs only in compounds. World production in 2000 was around 112,000,000 tons (CaO). Calcium is mined almost everywhere.
- Uses of Calcium:
- Used for dehydrating oils, decarburization and desulfurization of iron and its alloys, getter in vacuum tubes. Also used as an alloying agent for aluminum, copper and lead, a reducing agent for beryllium and used in fertilizer, concrete & plaster of paris. Calcium is an essential component shells, bones, teeth and plant structures.
- Additional Notes:
- Calcium was prepared as lime by the Romans under the name calyx in the 1st century A.D., but the metal was not discovered until 1808. Berzelius and Pontin prepared calcium amalgam by electrolizing lime in mercury. Davy was then successful in isolating the impure metal. Why did it take so long? Calcium is the fifth most abundant metalic element in the earth's crust, but is never found in the elemental form because it is so reactive. It is found in limestone (CaCO3) gypsum (CaSO4. 2H2O) and fluorite (CaF2). Pure calcium is a shiny soft metal that will react violently with water to produce hydrogen.
Calcium Menu
- Calcium Page One
- Calcium Page Two
- Calcium Page Three
References
A list of reference sources used to compile the data provided on our periodic table of elements can be found on the main periodic table page.
Related Resources
- Anatomy of the Atom
Answers many questions regarding the structure of atoms. - Molarity, Molality and Normality
Introduces stoichiometry and explains the differences between molarity, molality and normality. - Molar Mass Calculations and Javascript Calculator
Molar mass calculations are explained and there is a JavaScript calculator to aid calculations. - Chemical Database
This database focuses on the most common chemical compounds used in the home and industry.
Citing this page
If you need to cite this page, you can copy this text:
Kenneth Barbalace. Periodic Table of Elements - Calcium - Ca. EnvironmentalChemistry.com. 1995 - 2021. Accessed on-line: 4/24/2021
https://EnvironmentalChemistry.com/yogi/periodic/Ca.html
.
Linking to this page
If you would like to link to this page from your website, blog, etc., copy and paste this link code (in red) and modify it to suit your needs:
<a href='https://EnvironmentalChemistry.com/yogi/periodic/Ca.html'>echo Periodic Table of Elements: Calcium - Ca (EnvironmentalChemistry.com)</a>- Comprehensive information for the element Calcium - Ca is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions.
.
NOTICE: While linking to articles is encouraged, OUR ARTICLES MAY NOT BE COPIED TO OR REPUBLISHED ON ANOTHER WEBSITE UNDER ANY CIRCUMSTANCES.
PLEASE, if you like an article we published simply link to it on our website do not republish it.
Learning Objective
1. Draw a Lewis electron dot diagram for an atom or a monatomic ion.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. (It does not matter what order the positions are used.) For example, the Lewis electron dot diagram for hydrogen is simply
Because the side is not important, the Lewis electron dot diagram could also be drawn as follows:
The electron dot diagram for helium, with two valence electrons, is as follows:
By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1s subshell; this is the common convention we will adopt, although there will be exceptions later. The next atom, lithium, has an electron configuration of 1s22s1, so it has only one electron in its valence shell. Its electron dot diagram resembles that of hydrogen, except the symbol for lithium is used:
Beryllium has two valence electrons in its 2s shell, so its electron dot diagram is like that of helium:
The next atom is boron. Its valence electron shell is 2s22p1, so it has three valence electrons. The third electron will go on another side of the symbol:
Again, it does not matter on which sides of the symbol the electron dots are positioned.
For carbon, there are four valence electrons, two in the 2s subshell and two in the 2p subshell. As usual, we will draw two dots together on one side, to represent the 2s electrons. However, conventionally, we draw the dots for the two p electrons on different sides. As such, the electron dot diagram for carbon is as follows:
With nitrogen, which has three p electrons, we put a single dot on each of the three remaining sides:
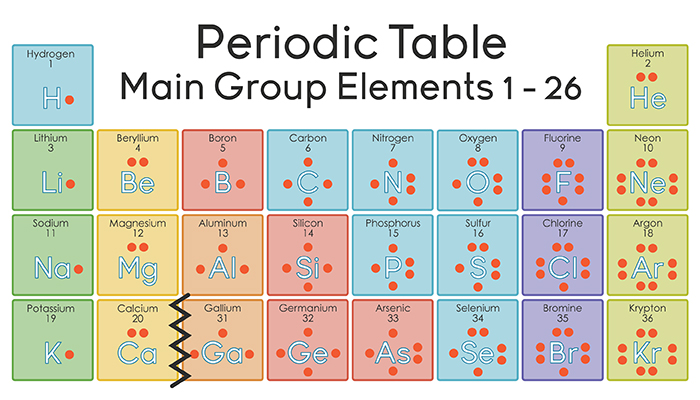
For oxygen, which has four p electrons, we now have to start doubling up on the dots on one other side of the symbol. When doubling up electrons, make sure that a side has no more than two electrons.
Fluorine and neon have seven and eight dots, respectively:
With the next element, sodium, the process starts over with a single electron because sodium has a single electron in its highest-numbered shell, the n = 3 shell. By going through the periodic table, we see that the Lewis electron dot diagrams of atoms will never have more than eight dots around the atomic symbol.
Example 1
What is the Lewis electron dot diagram for each element?
- aluminum
- selenium
Solution
The valence electron configuration for aluminum is 3s23p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons:
The valence electron configuration for selenium is 4s24p4. In the highest-numbered shell, the n = 4 shell, there are six electrons. Its electron dot diagram is as follows:
Test Yourself
What is the Lewis electron dot diagram for each element?
- phosphorus
- argon
Answer
For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4s23d6) is as follows:
Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration. Thus the electron dot diagrams for the first column of elements are as follows:
Monatomic ions are atoms that have either lost (for cations) or gained (for anions) electrons. Electron dot diagrams for ions are the same as for atoms, except that some electrons have been removed for cations, while some electrons have been added for anions. Thus in comparing the electron configurations and electron dot diagrams for the Na atom and the Na+ ion, we note that the Na atom has a single valence electron in its Lewis diagram, while the Na+ ion has lost that one valence electron:
Technically, the valence shell of the Na+ ion is now the n = 2 shell, which has eight electrons in it. So why do we not put eight dots around Na+? Conventionally, when we show electron dot diagrams for ions, we show the original valence shell of the atom, which in this case is the n = 3 shell and empty in the Na+ ion.
In making cations, electrons are first lost from the highest numbered shell, not necessarily the last subshell filled. For example, in going from the neutral Fe atom to the Fe2+ ion, the Fe atom loses its two 4s electrons first, not its 3d electrons, despite the fact that the 3d subshell is the last subshell being filled. Thus we have
Anions have extra electrons when compared to the original atom. Here is a comparison of the Cl atom with the Cl− ion:
Example 2
What is the Lewis electron dot diagram for each ion?
- Ca2+
- O2−
Solution
Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca2+.
Ca2+
The O2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows:
Test Yourself
The valence electron configuration of thallium, whose symbol is Tl, is 6s25d106p1. What is the Lewis electron dot diagram for the Tl+ ion?
Answer
Key Takeaways
- Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol.
- Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom.
Exercises
Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol.
Is it necessary for the first dot around an atomic symbol to go on a particular side of the atomic symbol?
What column of the periodic table has Lewis electron dot diagrams with two electrons?
What column of the periodic table has Lewis electron dot diagrams that have six electrons in them?
Draw the Lewis electron dot diagram for each element.
a) strontium
b) silicon
6. Draw the Lewis electron dot diagram for each element.
a) krypton
b) sulfur
7. Draw the Lewis electron dot diagram for each element.
a) titanium
b) phosphorus
8. Draw the Lewis electron dot diagram for each element.
a) bromine
b) gallium
9. Draw the Lewis electron dot diagram for each ion.
a) Mg2+
b) S2−
10. Draw the Lewis electron dot diagram for each ion.
Lithium
a) In+
Beryllium
b) Br−
11. Draw the Lewis electron dot diagram for each ion.
a) Fe2+
b) N3−
12. Draw the Lewis electron dot diagram for each ion.
a) H+
b) H−
Answers
1.
The first two electrons in a valence shell are s electrons, which are paired.
3.
the second column of the periodic table
5.
a)
b)
Cached
7.
a)
Ca Valence Electrons
b)
9.
a) Mg2+
b)
Ca^2+ Valence Electrons
11.
a) Fe2+
Ca Has How Many Valence Electrons
b)
